Abstract
Introduction: Folate deficiency has been widely reported in association with several physiologic and pathologic conditions such as pregnancy, malnutrition, chronic hemolysis, inflammatory bowel disease, alcoholism, and cancer, as well as in conjunction with the administration of several drugs and drug classes including anti-folates (e.g. methotrexate), anticonvulsants and contraceptives. (1) We identified new-onset severe folate deficiency in a patient receiving olaparib for treatment of relapsed ovarian cancer. Olaparib (Lynparza™) is a poly-ADP ribose polymerase (PARP) inhibitor approved for the treatment of relapsed ovarian cancer in BRCA 1 and 2 mutation carriers. It is reported to cause decreased hemoglobin in 90% of patients, with 15% experiencing grade 3-4 anemia. (2) The etiology of anemia in the setting of PARP inhibitor therapy is currently unknown. To our knowledge, this is the first report of severe folate deficiency induced by olaparib.
Methods: We performed a chart review of patients in our practice with relapsed ovarian cancer undergoing treatment with olaparib to determine the incidence of folic acid deficiency in this population. Patients found to be folate deficient were started on oral folic acid supplementation with close follow up. We assessed the response of folic acid supplementation on anemia. We also assessed the impact of anemia on olaparib dosing.
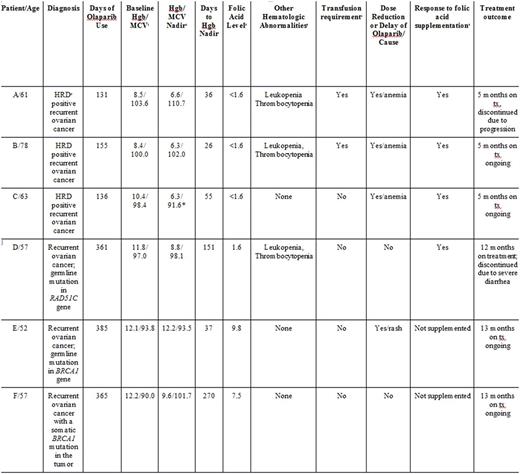
Results: We identified a total of six patients who were on active olaparib therapy for treatment of relapsed ovarian cancer. Five out of six patients developed a decrease in baseline hemoglobin after the initiation of olaparib. Four out of six patients (67%) had severe folate deficiency (<1.6 ng/mL, normal range 7-31.4 ng/mL) after taking olaparib for at least four weeks. None of these patients reported emesis to account for dietary folate deficiency. Three out of six patients required dose-reductions of olaparib due to the development of transfusion-dependent anemia, with a mean time of 39 days from the initiation of olaparib to hemoglobin nadir. In those found to have folate deficiency, folic acid supplementation was started at a range of 1 mg daily to 2 mg TID, with subsequent improvement of serum folate level and anemia. Subsequently, all four patients were able to continue olaparib therapy without further dose reductions and were also able to avoid additional blood transfusions.
Conclusion: In our small case series, we discovered that folate deficiency occurred in a majority of patients within weeks of initiating olaparib treatment. We plan to confirm our findings in a larger prospective trial in the coming months. We are also planning to study a potential association between PARP inhibition, BRCA mutations, and folate pathway. Once these findings are confirmed, we think that identifying and treating folate deficiency in this population is warranted, and may improve dose delivery and safety of olaparib therapy.
References:
1. Wani NA, Hamid A, Kaur J. Folate status in various pathophysiological conditions. IUBMB Life . 2008;60(12):834-842. doi:10.1002/iub.133.
2. Lynparza [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals, LP; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206162lbl.pdf. Published 2014. Accessed April 19, 2017.
Kuzel: Bristol Myers Squibb: Honoraria, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Eisai: Data monitoring committee honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria; Seattle Genetics Inc.: Membership on an entity's Board of Directors or advisory committees; Argos: Other: Data monitoring committee honoraria; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Exelexis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Medivation: Honoraria; Astellas: Honoraria; Sanofi: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Emmaus: Membership on an entity's Board of Directors or advisory committees; Amgen: Other: Data monitoring committee honoraria; Merck: Other: Data monitoring committee honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal